INSIGHT by the World Resources Institute
Fossil fuels aren’t just used to power cars, heat buildings and keep the lights on. They are, quite literally, woven into almost every facet of our lives.
From crayons, cosmetics and carpeting to fabrics, fertilizers and pharmaceuticals, around 70,000 everyday products are made with “petrochemicals” produced from fossil fuels. These products are so ubiquitous that many oil and gas companies are betting on chemical production to stay in business even as fossil fuel use in energy, heating and transport declines.
This comes with serious consequences for people and the planet. In the United States alone, chemical production directly emits 180 million tonnes of carbon dioxide equivalents (MTCO2e) per year — equivalent to the annual emissions from nearly 49 million gas-powered vehicles. The U.S. chemical sector also released 176,000 tonnes of toxic pollutants in 2021, exposing communities to water and air pollution as well as health risks like acute respiratory symptoms, skin and eye irritation and cancer.
One of the most important steps the industry can take to reduce these impacts is to replace fossil fuels used as ingredients in chemical products with non-fossil alternatives. This is known as “defossilization.”
While promising, defossilization technologies are rarely used at scale and face complicated hurdles. Some alternative materials are currently only available in small quantities. Others can risk increasing emissions if not used carefully. New analysis from WRI explores how and where U.S. chemical companies can use both existing and on-the-horizon technologies to reduce their reliance on fossil fuels, lower emissions and improve lives in nearby communities.
| How are fossil fuels used in everyday products?
“Petrochemicals” — chemicals derived from fossil fuels like petroleum, natural gas and coal — are present in just about every material that is not 100% organic, mineral or metallic. This includes plastics, electronics, textiles, cleaning products, rubber, paints and thousands of other synthetic products that most people use every day.
The process to make these products starts with processing fossil fuels into chemical “feedstocks” (or raw materials). Chemical feedstocks are turned into primary chemicals before being converted into intermediary chemicals and polymers. These are then manufactured into materials such as plastics and fibers and finally put to use in end products.
One of the most common chemical processing chains in the U.S. distills ethane from natural gas (a chemical feedstock), which is then “cracked” into ethylene (a primary chemical) and eventually turned into plastics and other materials.

Production of primary chemicals — including ethylene, propylene, benzene, toluene, xylene, ammonia and methanol — emits the most greenhouse gases along the chemical supply chain. These “process emissions” come from burning additional fossil fuels to generate the high temperatures (up to 1,000 degrees C) needed to turn fossil fuels into primary chemicals.
Ammonia, for example, is one of the most common chemicals globally due to its use in synthetic fertilizer. Producing it requires hydrogen, which is typically made by reforming natural gas into a mixture of hydrogen, carbon monoxide and carbon dioxide. The resulting CO2 is usually emitted into the atmosphere. Extracting and transporting natural gas to an ammonia plant also emits greenhouse gases and risks methane leakages. (Methane is a highly potent greenhouse gas with 80 times the warming power of CO2 over a 20-year period.)
Because this small handful of chemicals are the precursors to thousands of end products and drive most emissions in the product lifecycle, they offer a strategic emission reduction opportunity.
| How could fossil fuels be replaced in chemical production?
The modern chemical industry is built on fossil fuels because they are dense in energy as well as carbon and hydrogen (the two key molecules in most chemical products). This makes them an economical feedstock option. But, technically, anything containing many carbon and hydrogen atoms can be used to replace fossil fuels in chemical production.
WRI’s analysis considered the following alternative feedstocks that are either abundant today or are projected to be in the coming years:
- Electrolytic hydrogen: Pure hydrogen can be obtained by using electricity to split water (H2O) into hydrogen and oxygen through a process called electrolysis. This should be done using clean power to avoid adding greenhouse gas emissions from fossil-fueled electricity.
- Captured CO2: Carbon that is captured from industrial sources (such as cement manufacturers), or from the atmosphere (via direct air capture and other methods) could be used in chemical production.
- Waste biomass: This includes unused plant parts and other organic material collected in agriculture, forestry and municipal waste. Waste biomass can be a substitute for fossil fuels because, technically, fossil fuels are just biomass and animal matter subjected to heat and pressure underground for millions of years; both contain the same carbon and hydrogen molecules. It is important that biomass truly comes from waste and is not purpose-grown for the chemical industry, as converting carbon-rich natural ecosystems to cropland can drive enormous land-use-change emissions.
- Ethanol: Ethanol, which is currently widely produced in the U.S. by fermenting corn, can be used in place of fossil fuels to produce the chemical ethylene. While there is an opportunity cost of using prime farmland for corn ethanol, using ethanol as a chemical feedstock is more productive than blending it with gasoline as a “renewable” fuel. Capturing the CO2 emitted during ethanol production would reduce emissions from existing facilities.
Consider ammonia once more. Rather than deriving hydrogen from natural gas, an ammonia plant can defossilize by using electrolysis to split water into its component hydrogen and oxygen molecules. Electrolysis does not emit greenhouse gases if the electricity comes from zero-carbon sources like wind or solar and does not displace clean energy used elsewhere on the electricity grid. Because most of the emissions caused by ammonia production derive from reforming natural gas, replacing it with clean hydrogen makes the process nearly zero-carbon.
| Opportunities to defossilize chemical production in the U.S.
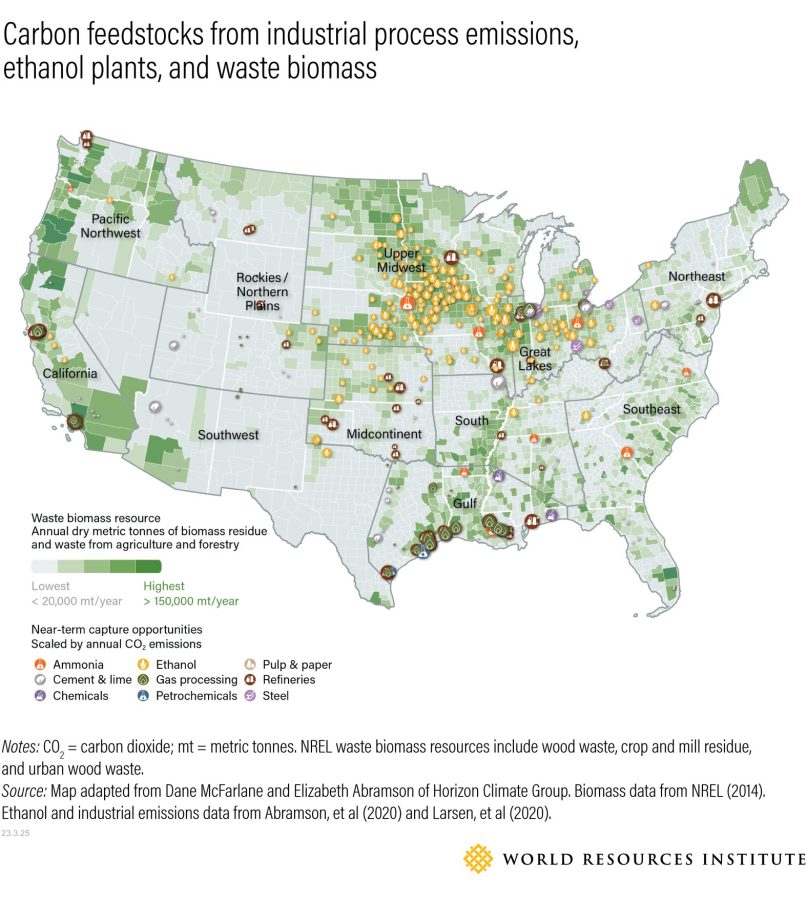
New WRI analysis looks at defossilization opportunities in the U.S. for four primary chemicals: ethylene, propylene, ammonia and methanol. It assesses total demand for each, identifies today’s most promising defossilization technologies and estimates the volume of these feedstocks needed to meet demand. It also maps out where alternative feedstocks are or could be located effectively in relation to existing chemical plants and infrastructure.

We found that, nationally, the estimated demand for alternative feedstocks is currently greater than available feedstock supplies. In some cases, the difference is relatively small: The U.S. currently produces around 315 million tonnes of waste biomass per year, and the estimated demand for chemical production is around 375 million tonnes. In other cases, demand massively outstrips supply. For example, as much as 29-41 million tonnes of electrolytic (clean) hydrogen would be needed as a chemical feedstock. The U.S. currently produces almost none, although this is expected to change thanks to recent production incentives. While the U.S. produces 10-11 million tonnes of conventional (dirty) hydrogen, this would not be a low-carbon feedstock.
The outlook is different from a regional perspective, however. In certain areas with a small amount of chemical production, demand could be easily met by a large supply of potential resources.
| Using renewable energy to make ammonia in the Midwest
The Midwest is home to 127 million acres of farmland, much of which produces corn used for ethanol and for feeding livestock (45% and 40% of corn crop, respectively) as well as soybeans and other food crops. This immense agricultural output relies on millions of tons of ammonia-based fertilizer made with natural gas feedstock. To meet this demand, most U.S. ammonia plants are sprinkled throughout the region, with additional demand met by shipments produced in the Gulf Coast. We estimate that the Midcontinent, Great Lakes and Upper Midwest regions combined — which make up most of the country’s “corn belt” — produce about half the country’s ammonia (6.9 million tons annually). Replacing natural gas feedstocks in this process with clean, electrolytic hydrogen would require about 1.2 million tons of hydrogen per year. Fortunately, the Midwest also has some of the United States’ best wind energy potential and respectable solar potential. Depending on the electrolyzers’ efficiency, creating 1.2 million tons of electrolytic hydrogen would require 42-62 thousand gigawatt hours (GWh) of clean electricity. This is about 7%-11% of the total renewable energy the Midwest could generate in 2050 with a 95% decarbonized energy grid.
Defossilizing ammonia in the Midwest may need both demand and supply side solutions. To avoid using up to 11% of the region’s renewable energy, one option would be to transition just half of the Midwest’s ammonia production to hydrogen made with renewable electricity. This would reduce around 7.5 MT of CO2 emissions annually, equal to taking about 1.5 million gas-powered cars off the road for a year. It is also possible that this ammonia demand could fall if corn crops grown for ethanol fuel production decrease as ground transportation electrifies, lowering the size of the challenge.
Defossilizing chemical production in the Gulf Coast
The largest regional hurdle is defossilizing the Gulf Coast, which produces over half of the United States’ primary chemicals. Still, it has significant feedstock resource potential, with the highest CO2 process emissions, second highest projected renewable generation in 2050, and fifth highest volume of waste biomass of any region in the U.S. There are also existing ethanol transport networks linking the Midwest to the Gulf Coast. In other words, companies would have some flexibility in selecting which pathways they would use to defossilize their production rather than all competing for one feedstock.
| What will it take to defossilize U.S. chemical production?
Defossilizing all U.S. chemical production will be a multi-decade undertaking. It will require massive effort and investment from both the government and private sector as well as measures to uplift communities impacted by chemical plants.
Overcoming technology hurdles
While some defossilization methods are already commercially viable, sustainable supplies of feedstocks like waste biomass and clean hydrogen are limited. Carbon capture technology needs more private investment and deployment. Renewable energy generation, required for clean hydrogen, is already pacing behind what’s needed in a net-zero economy. And competition for resources like clean electricity would put the chemical sector at odds with other sectors seeking to reduce emissions.
Other technologies, like direct air capture, have not yet been demonstrated at a sufficient scale but are poised to be within the decade.
Retrofitting existing chemical plants and building new plants and infrastructure would also require extraordinary effort. Financing new technologies, re-engineering existing facilities to accommodate new equipment, and permitting and building clean energy and energy infrastructure — such as transmission lines, CO2 and hydrogen pipelines — would likely be the largest obstacles.
However, existing policy opportunities can help clear these hurdles. The Bipartisan Infrastructure Law (BIL) and Inflation Reduction Act (IRA) made billions of dollars of government funding available for industrial decarbonization. Several of the programs these laws established could be used to defossilize chemicals, including tax credits for clean hydrogen, carbon utilization and energy storage; grants for first-of-a-kind low-emission commercial and demonstration facilities; and research and development funding.
Making sure benefits flow to affected communities
Defossilization can also provide some social and health benefits by reducing local pollution. Communities located near chemical production facilities have long been affected by air and water pollution, leading to above average rates of cancer, respiratory illness, infertility and natal issues, among other health problems. “Sacrifice zones” with persistent structural inequality due to environmental damage and poor economic investments, like Louisiana’s “Cancer Alley,” also see pervasive poverty.
For some in those communities, shutting down chemical facilities might be the only acceptable solution. But others might view plants as a source of jobs that would not exist if facilities shut down. Defossilization could provide a middle ground here. For example, electrifying some chemical processes with renewable energy could keep facilities operating and local people employed while eliminating processes that burn fossil fuels and cause local air pollution.
Recent equity-focused policies in the U.S. — such as the Biden Administration’s Justice40 initiative — can help ensure that benefits like new jobs reach community members. In many cases, projects funded by the IRA or BIL must submit community benefit plans outlining how the investments will benefit nearby communities from an economic, health and/or environmental standpoint. While initiatives like this are a step in the right direction, they should be only the foundation for further action.
Strengthening policy support at the federal and local levels
Strong federal and state policy can help defossilize chemical production. Policymakers will need to maintain or expand existing incentives like tax credits, loans and grants for decarbonization that can help finance fuel switching and new technologies. Other policies can help stimulate demand for clean chemicals, including procurement programs and contracts for differences. Emissions caps and carbon taxes are ways to compel companies to change and would provide greater certainty for the environment and the market. Paired with carbon trading markets, these policies can also provide financing for the transition.
Finally, policy shifts such as clean energy permitting reform and increased support for research and development are critical to maximize the potential of decarbonization incentives. While some of these policies are more politically challenging to pass than others, a combination of them will be needed to get defossilization off the ground.
| It’s time to stop ignoring the chemical industry
The chemical sector has received relatively little attention in climate discussions to date. Yet, its large emission impacts and ubiquitous presence mean the sector urgently needs to change. Chemical producers have many available and near-term tools to reduce emissions and clean up their manufacturing processes, and defossilization will be key among them.
Removing fossil fuels from chemical production to the greatest extent possible, just as in other sectors, will be pivotal to both meeting U.S. climate goals and advancing the health and well-being of communities.
| All opinions expressed are those of the author and/or quoted sources. investESG.eu is an independent and neutral platform dedicated to generating debate around ESG investing topics.









